Membrane processes, using the electric potential gradient as the driving force of transport, are called ELECTROMEMBRANE PROCESSES. In these processes, so-called ion-exchange or bipolar membranes are applied as the basic elements of the entire process.
-
Based on the type of fixed ion-exchange groups, ion-exchange membranes are divided into:
-
Cation-exchange membranes (CM)
-
They contain acid ion-exchange groups with negative electric charge (-SO3-, COO-) bound in a polymeric matrix, allowing free passage only of particles with positive charge, because passage of particles with negative charge is substantially limited.
-
-
Anion-exchange membranes (AM)
-
They contain basic cation-exchange fixed groups (-NR3+, where R is hydrogen or alkyl group), allowing free passage only of particles with negative charge, because passage of particles with positive charge is substantially limited (conversely to cation-exchange membranes).
Advantages of electromembrane processes:
-
high efficiency of separation of substances without phase transitions,
-
easy continualization and automation, low demands for space,
-
lower energy consumption, compared to traditional procedures,
-
possibility to create closed waste-free technological junctions or entire technologies.
Disadvantages of membrane processes:
-
removal of so-called membrane poisons by pre-treatment of solutions,
-
fouling (creation of coating of poorly soluble substances on the membrane surface).
The overview of the most important and widely used electromembrane processes is given in the table.
|
Driving force |
Process name |
|
electric potential gradient |
electrodialysis (ED), electrophoresis (EF), electrodeionization (EDI), membrane electrolysis (ME) |
-
Electrodialysis
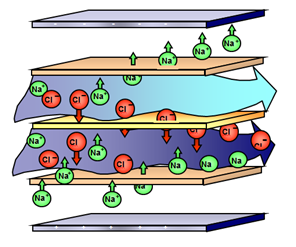
Principle: The separation of substances is achieved by the influence of electric field and on the basis of varied permeability of ion-exchange membranes for individual ingredients of the solution. The basic arrangement of the electrodialysis stack is shown in Figure 1.
Cathode (-)
Electrode solution
Cation-exchange membrane (CM)
Diluate
Anion-exchange membrane (AM)
Concentrate
Cation-exchange membrane (CM)
Electrode solution
Anode (+)
(Fig 1)
Typical application of electrodialysis:
-
Desalination of underground, brackish or sea water in production of drinking water,
-
Production of saline concentrates of sea, waste or industrial water/solutions,
-
Refining of waste and industrial water (recycling and regeneration),
-
Separation of organic and inorganic substances (electrolyte/non-electrolyte),
-
Demineralization of whey.
-
Electrophoresis
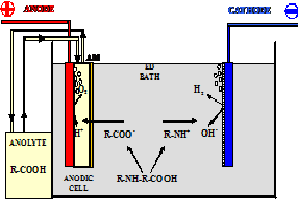
Principle: In electrophoresis, a positive (cataphoresis) or negative (anaphoresis) part of the dissociated paint is applied on metal materials by means of the application of an ion-exchange membrane in the electric field. The basic principle is shown in Figure 2.
At present, cataphoresis is the most widely used, modern and highly effective industrial method of complete surface treatment of metal parts, using the electric potential. Cataphoresis is applied where good anti-corrosion protection of metal surfaces is required.
(Fig 2)
Particular application:
In applications, an ion-exchange membranes placed in so-called electrophoretic box is used. These boxes can be either plane or tubular. At present, tubular boxes are widely preferred for easier handling and maintenance, and to minimize production losses.
Tubular box Plane box Tubular box in the bath Production machine




Tubular box machine Plane box Tubular box in the bath Production machine
Typical applications of electrophoresis
Automotive industry, consumer industry, electronics.
-
Electrodeionization
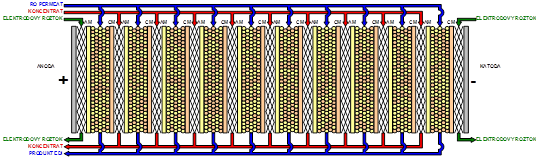
Principle: The basic scheme is shown in Picture 4.
EDI combines electrodialysis (ED) with ion-exchangers in one device. Similarly to ED, the process module consist of a raw of alternating anion-exchange (AM) and cation-exchange (CM) membranes, separated by membrane spacers and placed between a pair of binding desks, equipped with electrodes on their inner sides. Spacers define the space for the flow of the liquid between membranes and ensure the liquid distribution between individual flow chambers and sealing of the device. The chambers closed with the anion-exchange membrane on the anode side and with the cation-exchange membrane on the cathode side are called the diluate chambers. In EDI, the diluate chambers are filled with the mixed bed of ion-exchangers, i.e. a mixture of cation-exchanger and anion-exchanger in a certain ration. The liquid flowing through the diluate chambers during operation is called the diluate. The chambers closed with the anion-exchange membrane on the anode side and with the cation-exchange membrane on the cathode side are called the concentrate chambers.
(Fig. 4)
Hydraulic scheme of the EDI module. AM – anion-exchange membrane, CM – cation-exchange membrane, diluate chamber are closed with AM on the anode side and CM on the cathode side and filled with a mixture of cation-exchanger and anion-exchanger (mixed-bed); concentrate chamber are closed with CM on the anode side and AM on the cathode side and filled with a plastic net; electrode chambers are the chambers adjacent to electrode and filled, similarly to the concentrate chambers, with a plastic net.
Typical use of electrodeionization
Preparation of purified water over 5mΩ, primarily for power engineering, pharmaceutical industry and semiconductor industry.
-
Membrane electrolysis
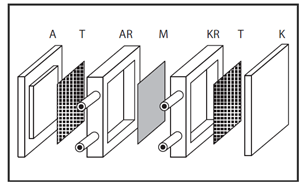
Principle: The basic principle is shown in Figure 5.
(Fig. 5)
Scheme of a electrolytic cell of the filter press type
A – anode, T – turbulizer to increase the flow turbulence,
AR – frame of the anode space,
M – membrane, KR – frame of the cathode space,
K – cathode.
A typical and the most widely used example of the industrial application is the production of chlorine and sodium hydroxide by means of the electrolytic solution of a sodium chloride solution.


































 EUROPEAN UNION
EUROPEAN UNION